Filter
Showing all 2 results
-
 HCP
HCPInstillagel®
Quantity: 1Sterile gel containing a local anaesthetic and antiseptic presented in a sterile package.View product View -
 HCP
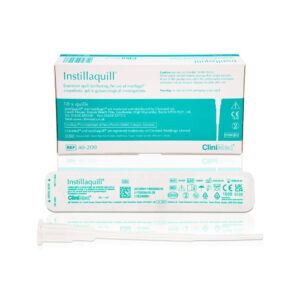
HCPInstillaquill®
Quantity: 1Single use extension tube which facilitates the use of Instillagel anaesthetic gel in gynaecological investigations.View product View


