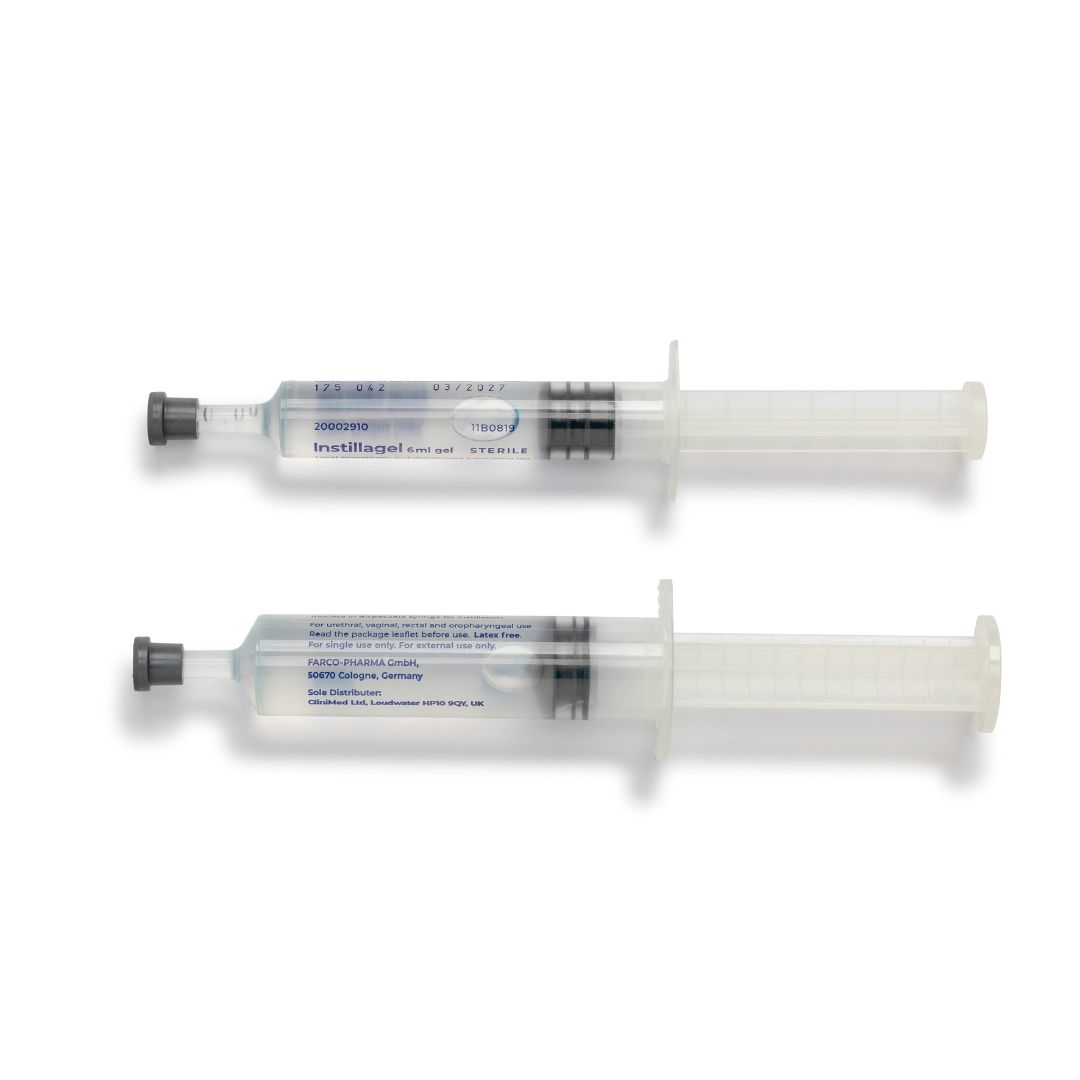
About
Active ingredients
Each 100 grams contains:
- Lidocaine Hydrochloride (Local anaesthetic) 2.000g
- Chlorhexidine Gluconate Solution (Antiseptic) 0.250g
- Methyl Hydroxybenzoate (E218) (Antiseptic) 0.060g
- Propyl Hydroxybenzoate (E216) (Antiseptic) 0.025g
In a gel made with Hydroxyethylcellulose, Propylene Glycol and Purified Water. The gel comes in disposable syringes each containing either 6ml or 11ml.
Before Instillagel is used
Tell the person preparing to use the gel if any of the following applies to you:
Do not use Instillagel:
- If you are allergic (hypersensitive) to lidocaine, chlorhexidine, methyl hydroxybenzoate, propyl hydrobenzonate or any of other ingredients of Instillagel
- If the moist lining of the application site is damaged or bleeding
Care should be taken when using Instillagel:
- If you have heart problems
- If you have liver problems
- If you are epileptic
Pregnancy and breastfeeding
Tell the person preparing to use the gel if you are pregnant or think you might be pregnant. You can continue to breast-feed.
Has the gel any side effects?
You might feel a slight stinging just after the gel is used, but this stops as soon as the anaesthetic starts to work. Most people find that there are no problems after the gel has been used but there may be a slight soreness when the effect of the local anaesthetic has worn off. If you feel that you have had any reaction to the gel, please tell your doctor as soon as possible.
Features
• Local anaesthetic and antiseptic action, providing pain relief and reducing infection
• Excellent and long-lasting lubricating properties
• Sterile, ready-to-use syringe is easy to handle
• Available in 6ml and 11ml syringes
• P Licensed medicine ensures Instillagel always has the correct levels of active pharmaceutical ingredients
• Steam sterilising can help to reduce the degradation of the active ingredients1
1N. Steiner-Reischutz, Michael Pyerinet al, Evaluating the Impact of Sterilization on Gel Formulations. Pharmaceutical Technology Vol 42 (4) 2018.
Usage
Please ensure to read the full Instructions for Use before using this product.
Read the patient information leaflet.
How Instillagel is used
Instructions for use are provided on the product packaging. The gel is available in two sizes – 6ml and 11ml. Usually the complete contents of the size suitable for the procedure will be used. The syringe is removed from its sterile package by tearing off the backing paper. Before removing the grey cap from the end of the syringe, free the plunger by gently pressing it. Remove the cap. Insert the nozzle into the opening of the area to be anaesthetised and press the plunger slowly to push out the gel. The anaesthetic takes about 5 minutes to work after the gel has been used.
After using the gel
You may feel drowsy after using Instillagel, if so do not drive or use machinery. If the gel has been used orally (inserted into your mouth), care should be taken when chewing or swallowing as you may bite yourself without realising.
Storage of the gel
The gel should not be used after the expiry date shown on the package. Instillagel does not require any special storage conditions. The syringe is for single use only. If the complete contents are not used, the syringe and remaining gel must be thrown away.
As with all medicines, keep Instillagel away from children.
Read the summary of Product Characteristics.