Designed to free up clinic time, and empower patients to manage treatment at home.
Managing Overactive Bladder (OAB) can be complex. Medications are often ineffective or poorly tolerated. Bladder interventions such as Botox or Percutaneous Posterior Tibial Nerve Stimulation (P-PTNS) require specialist input, with long waiting times to be seen.
Tensi+ helps clinicians by addressing long NHS waiting times, aligning with NHS priorities for efficiency, sustainability, and patient-centred care.
Discover how Tensi+ works, explore the latest safety and efficacy data from recent multicentre studies, and learn how Tensi+ can deliver meaningful benefits for both patients and the NHS.
Supporting you and your patients
Optimised
Stimulates the posterior tibial nerve at the correct anatomical site.
Effective
Validated in published multicentre clinical studies, showing 67% efficacy with high levels of patient satisfaction.1
Simple
Simple for patients to use independently, supporting compliance and sustained outcomes.
How Tensi+ Works
Practical use
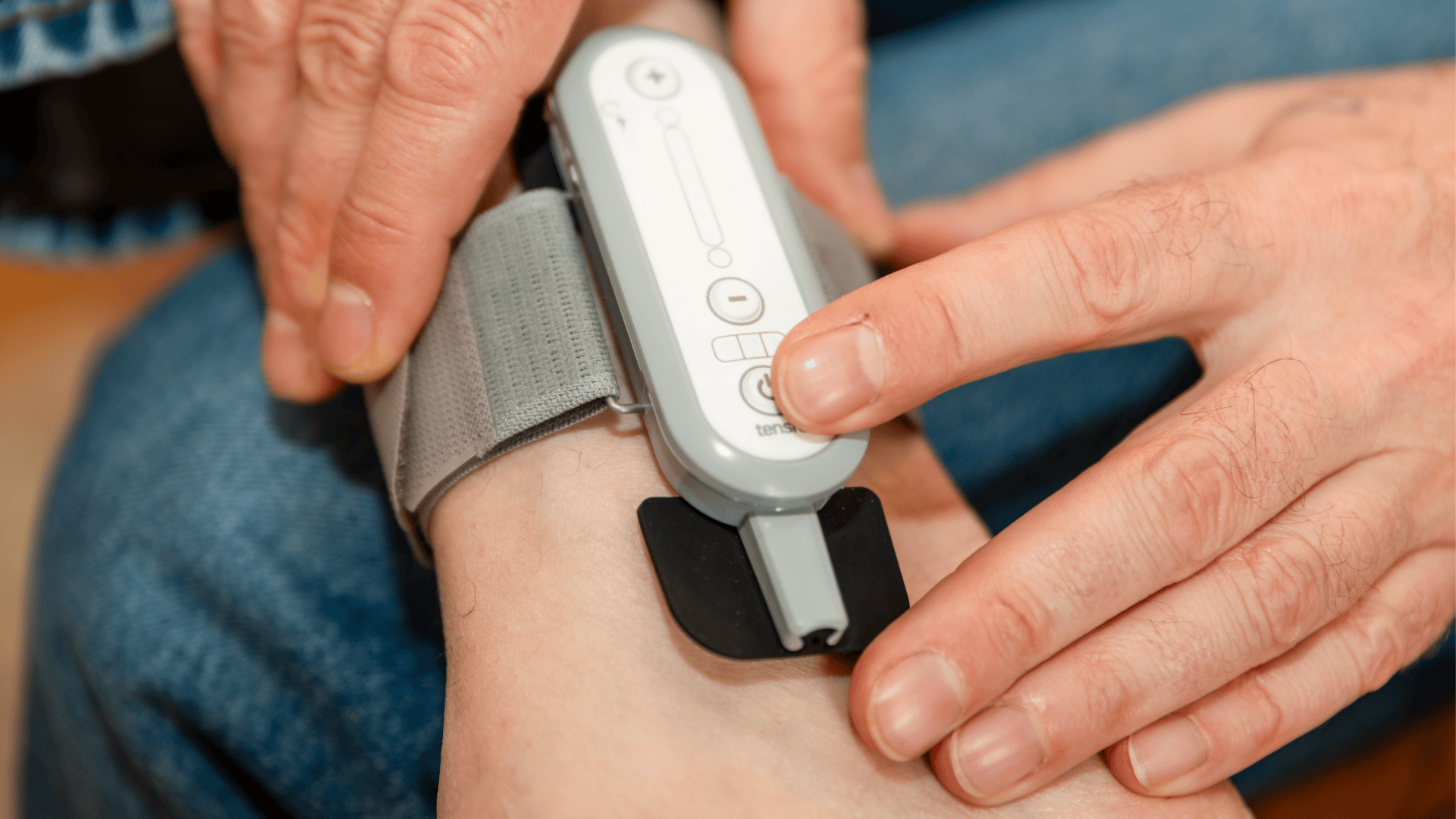
Tensi+ is a CE-marked, MHRA-registered medical device designed specifically for the treatment of OAB using Transcutaneous Posterior Tibial Nerve Stimulation (T-PTNS).
20 mins a day for 12 weeks, for the initial treatment protocol
Simple device placement, fixed electrode positioning
Self-management reduces follow-up burden

Benefits for the NHS
Clear cost savings versus pharmacological and invasive interventions
Aligns with Getting It Right First Time (GIRFT) and MedTech initiatives
Supports Value-Based Procurement by reducing pathway costs, not just product cost
Contributes to NHS Net Zero targets by reducing hospital appointments
Minimises follow-up burden with patient self-management

Who Tensi+ is for
Tensi+ is suitable for adults (18+). However, Tensi+ should not be used if you:
- Have a pacemaker, defibrillator, or any other electronic implant (risk of electric shock, malfunction, burns, or interference)
- Have a metal implant near the area of stimulation
- Have ankle joint issues, swollen ankles, a skin condition, or damaged skin at the electrode site
- Are pregnant
- Have a cognitive impairment
Clinical evidence and endorsements
Tensi+ is endorsed by leading urologists. There are also recent multicentre studies confirm Tensi+ is effective and safe in the management of OAB.
67% patient efficacy after 3 months1
88% patient satisfaction, with high usability and tolerability1
Comparable outcomes to P-PTNS and Botox, without the need for repeated clinical appointments

Clinical Case Study
Breaking Free From Overactive Bladder: Real-life experience of five patients who successfully used Tensi+ to manage OAB symptoms.
European Urology Focus
New TENSI+ Device for Transcutaneous Posterior Tibial Nerve Stimulation: A Prospective, Multicentre, Post-market Clinical Study.
The French Journal of Urology
Efficacy and Safety of the TENSI+ Device For Posterior Tibial Nerve Stimulation: A Multicenter, Retrospective Study.
The Canadian Journal of Urology
How I Do It: Transcutaneous Tibial Nerve Stimulation TENSI+ System.
Testimonials

I have more confidence when out and about and my sleep is improved.
Mrs J W – Hampshire

My life is no longer controlled by knowing where the toilet is.
Mr C B – Hampshire

Thanks to Tensi+ I’ve had the best 6 weeks for years.
Mr R D – London
Introduce Tensi+ into your service
How to order
You can purchase Tensi+ directly from CliniMed. Simply submit your details below and our team will be in touch.
If you’d like to add Tensi+ to your service and purchase it through the NHS Supply Chain, please contact your Trust’s procurement team and follow the steps below to place an order:
Your procurement team emails NHS Supply Chain stating their intention to buy Tensi+ and the Tensi+ gel via DPS
NHS Supply Chain provides a Statement of Requirements (SoR) form
Procurement completes and returns the SoR. NHS Supply Chain will obtain a supplier quote from CliniMed for the number of units they require and issue a Purchase Order (PO)
Tensi+ is delivered directly to your Trust
Your hospital is invoiced by NHS Supply Chain, and payment is processed through the usual internal process

References
- J.-N. Cornu, L. Donon, C. Thullier et al., New TENSI+ Device for Transcutaneous Posterior Tibial Nerve Stimulation: A Prospective, Multicentre, Post-market Clinical Study, Eur Urol Focus (2024), https://doi.org/10.1016/j.euf.2024.05.013




